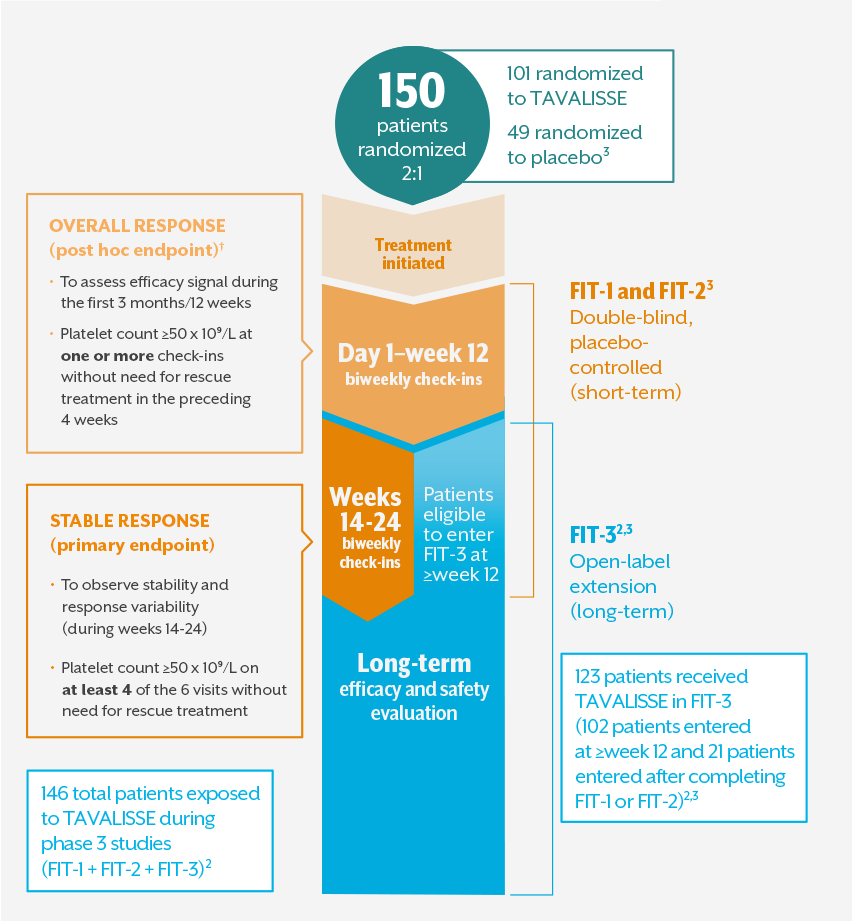
THE FOSTAMATINIB IN ITP (FIT) PROGRAM WAS DESIGNED TO EVALUATE SHORT- AND LONG-TERM TREATMENT EFFECTS
The FIT-1, FIT-2, and FIT-3 phase 3 clinical trials assessed TAVALISSE efficacy, safety, and durability1,2,*
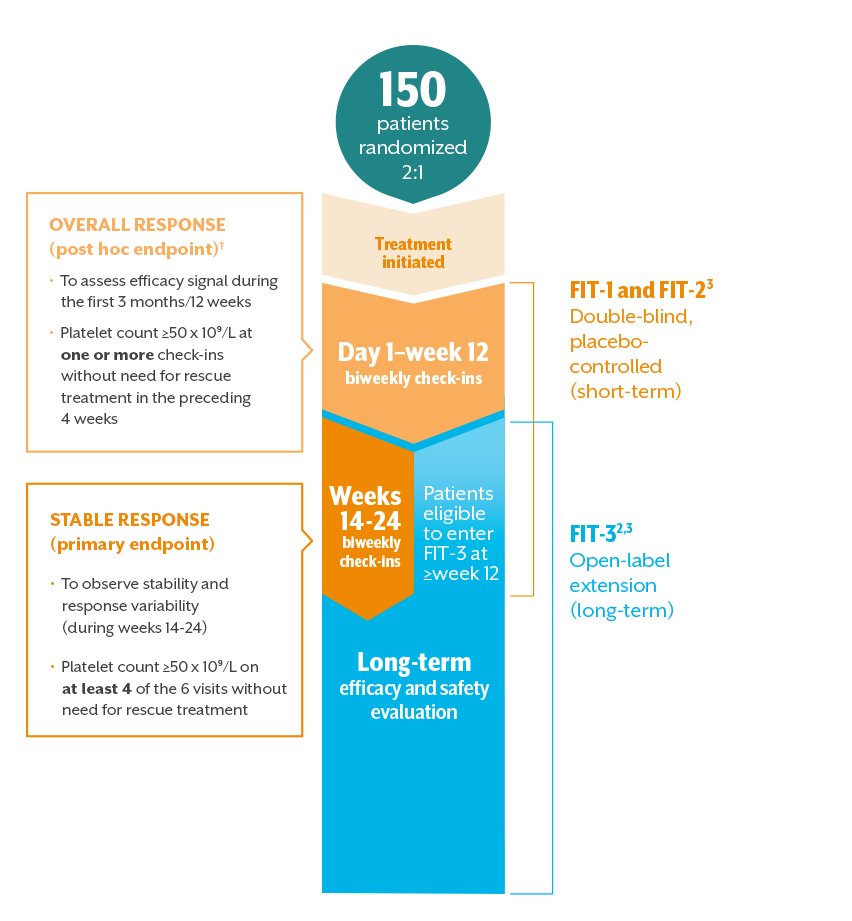
- All patients with platelets <50 x 10⁹/L, regardless of treatment arm, were eligible to enter FIT-3 (open-label extension) at or after week 121
- In clinical trials, stable concurrent ITP therapy was allowed, and rescue therapy was permitted if needed. Visits where rescue medication was used were excluded from the efficacy analysis3,‡
*Duration of response analysis endpoint: the number of days from achieving a platelet count ≥50 x 10⁹/L to reaching a platelet count <30 x 10⁹/L at two consecutive visits. Patients were classified as losing response if platelet counts dropped below 30 x 10⁹/L for two consecutive visits 28 days apart, or if rescue medication was used.2
†Overall response: the initial 12 weeks were selected since most nonresponding patients from both arms discontinued FIT-1 and FIT-2 and entered the open-label extension study at or very soon after week 12.
‡Stable concurrent ITP therapies permitted in clinical trials: glucocorticoids (<20 mg prednisone equivalent per day), azathioprine, or danazol.
THE FIT PROGRAM INCLUDED ADULT PATIENTS WITH CHRONIC ITP WHO HAD RECEIVED ≥1 PRIOR THERAPY
FIT program patients were representative of the general adult chronic ITP population, including exposure to prior therapies
Select baseline patient characteristics in FIT-1 and FIT-23,4
| Median time since diagnosis | 8.5 years |
| Median age | 54 years |
| Gender | 61% female; 39% male |
| Median platelet count | 16 x 109/L |
| Platelet counts <15 x 109/L | 45% |
| Splenectomy (time since splenectomy) | 35% (all >6 months, except for 1 patient) |
Prior treatment exposure among patients in FIT-1 and FIT-21,3,4
| Median number of prior therapies | 4 |
| Corticosteroids | 94% |
| Immunoglobulins | 53% |
| TPO-RAs | 48% |
| Immunosuppressants | 44% |
| Rituximab | 32% |
TAVALISSE dosing3
- Starting dose of TAVALISSE: One 100 mg tablet BID
- Based on platelet count and tolerability, the dose could be increased to one 150 mg tablet BID at or after week 4
- Patients who had a platelet count ≥50 x 109/L at the time of rollover to the FIT-3 open-label extension study maintained their existing dose of TAVALISSE
References: 1. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. 2. Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546-553. 3. TAVALISSE® [package insert]. South San Francisco, CA: Rigel Pharmaceuticals, Inc.; April 2018. 4. Data on file, Rigel Pharmaceuticals, Inc. April 2018.