79-YEAR-OLD MALE WITH CHRONIC ITP AND PERIPHERAL VASCULAR DISEASE

George*
BACKGROUND
| AGE: |
79
|
| OCCUPATION: |
Retired
|
| DATE OF DIAGNOSIS: |
2010
|
- Peripheral vascular disease; controlled with aspirin 81 mg QD and clopidogrel 75 mg QD
- Hypertension; now controlled with metoprolol tartrate 50 mg BID and losartan/hydrochlorothiazide 50/12.5 mg QD
- Hyperlipidemia; controlled with simvastatin 20 mg QD
- Diabetes mellitus type 2; controlled with diet
- Atrial fibrillation diagnosed 10+ years ago; had Watchman procedure in January 2019
- Platelet Counts
- Treatment Summary
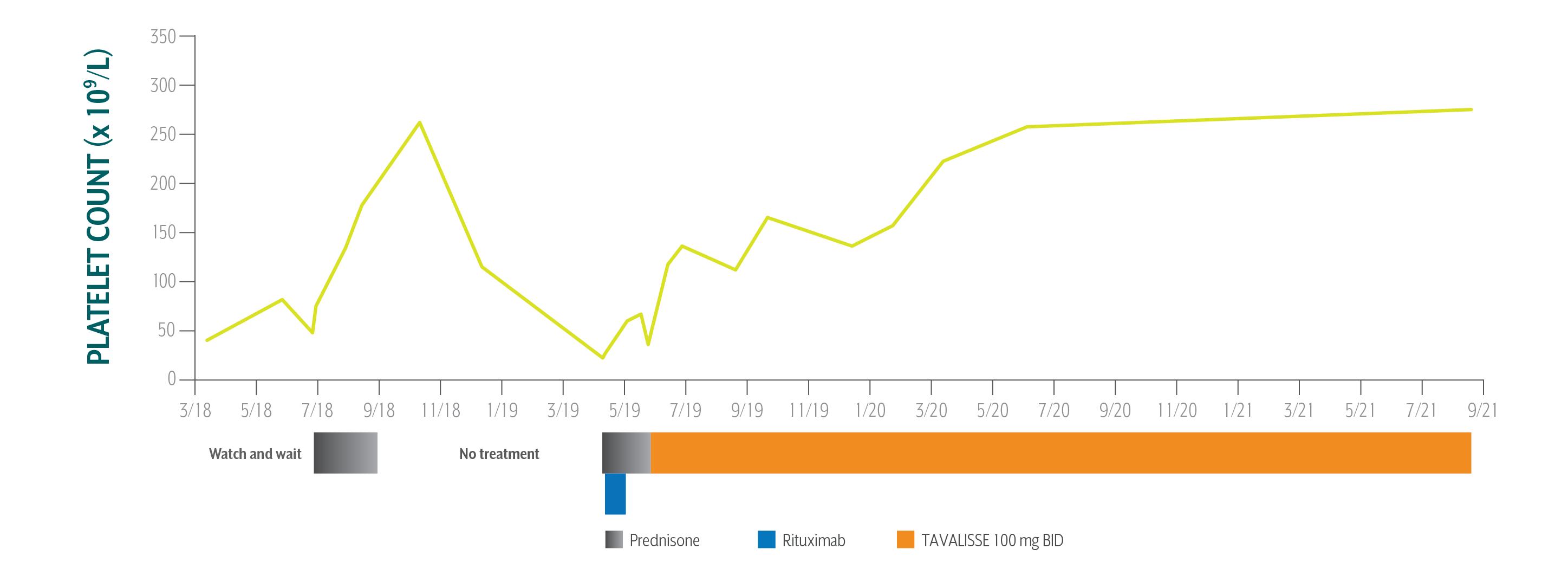
George's Platelet Counts Over Time
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
The patient has been on TAVALISSE for over 2 years and is very happy with his response. His platelet counts increased consistently, and in a stable fashion. He has not had any bleeding episodes and his petechiae has resolved.
AE=adverse event; ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; LFT=liver function test; TPO-RA=thrombopoietin receptor agonist; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
George's Treatment Summary
- Headings:
Prior Treatment history
March 12, 2018: Cardiologist refers patient to hematology/oncology (hem-onc) specialist to reduce bleeding risk with anticoagulant therapy
- Headings:
treatment with tavalisse
May 23, 2019: TAVALISSE initiation
- Platelet Count:
40 x 109/L
- Platelet Count:
36 x 109/L
- Clinical Observations:
- Epistaxis, petechiae, and fatigue
- Clinical Observations:
- Epistaxis, purpura on lower extremities, petechiae
- Laboratory Findings:
- Within normal limits (WNL)
- Laboratory Findings:
- BP: 133/62 mm Hg
- WBC: 9.5 x 109/L
- Glucose: 203 mg/dL
- AST: 17 IU/L
- ALT: 20 IU/L
- Bilirubin: 0.6 mg/dL
- Patient Discussion:
- Patient expresses anxiety about bleeding risk with his treatment for atrial fibrillation and peripheral vascular disease
- Patient Discussion:
- Physician explained the need for a treatment that could help improve platelet counts so that patient could continue proper anticoagulation therapy
- Physician discussed several options with the patient, including splenectomy, TPO-RAs, and TAVALISSE, and reviewed potential efficacy and AEs for each treatment. Patient did not like the idea of splenectomy
- Physician wanted to avoid therapies with a risk of bone marrow fibrosis or thromboembolism. Based on the patient’s cardiovascular issues, TPO-RAs were ruled out
- Treatment Plan:
- Cardiologist and hem-onc confer on treatment and decide to take a “watch and wait” approach while maintaining a platelet count ≥50 x 109/L
- Treat with steroids if platelet count drops below 50 x 109/L
Based on George’s response to prior therapies, comorbidities, and higher risk of bleeding, the hem-onc sought an oral medication in a different class of therapy.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and increase to 150 mg BID after week 4 if necessary
- Continue aspirin and clopidogrel for coronary and peripheral artery disease
The patient has been on TAVALISSE for over 2 years and is very happy with his response. His platelet counts increased consistently, and in a stable fashion. He has not had any bleeding episodes and his petechiae has resolved.
AE=adverse event; ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; LFT=liver function test; TPO-RA=thrombopoietin receptor agonist; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

DUE TO CONCERNS OF BLEEDING WITH ANTICOAGULATION THERAPY, CARDIOLOGIST REFERRED PATIENT TO HEMATOLOGY/ONCOLOGY SPECIALIST (HEM-ONC)