Use these materials to familiarize yourself with information to confidently prescribe TAVALISSE.
TAVALISSE RESOURCES

Prescribing Information
Information such as indication, dosing, safety, and efficacy as approved by the FDA.

Dosing and Administration Guide
Learn about the convenience of oral dosing without food restrictions, dosing modifications, and management of certain adverse reactions.

TAVALISSE placebo-controlled trials (FIT-1 + FIT-2) in the American Journal of Hematology
Review the published results of FIT-1 and FIT-2 in: Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930.

TAVALISSE open-label extension study (FIT-3) in the American Journal of Hematology
Review the published results of FIT-3 in: Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546-553.

Post hoc analysis of the TAVALISSE trial population by line of therapy in the British Journal of Haematology
Review the published post hoc analysis of the FIT studies in: Boccia R, Cooper N, Ghanima W, et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol. 2020;190(6):933-938.

Post hoc long-term thrombotic risk and efficacy analysis in Therapeutic Advances in Hematology
Review the published post hoc analysis of the FIT studies in: Cooper N, Altomare I, Thomas MR, et al. Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12:e20406207211010875. doi:10.1177/20406207211010875

Distribution Information
Find information on TAVALISSE distribution and pharmacy networks and patient support services
Take a closer look at TAVALISSE—learn more about ITP pathogenesis, SYK inhibition, and a patient's experience with treatment.

Hear from Dr Craig Kessler
Professor of Medicine and Pathology with the Division of Hematology-Oncology, Director of the Division of Coagulation in the Department of Laboratory Medicine at the Georgetown University Medical Center

Hear from ITP experts and a patient living with chronic ITP
Mohit Narang, MD: Director, Maryland Oncology Hematology
Adam Schmuckler, PA-C, MMSC: Maryland Oncology Hematology
Connie: a patient living with chronic ITP
Learn about Connie's treatment journey in an in‑depth conversation with her healthcare team:
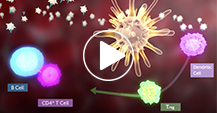
Mechanism of Action
See how TAVALISSE is believed to inhibit downstream signaling that leads to platelet destruction in chronic ITP.
These resources are designed to provide key points of support to patients all the way through their TAVALISSE treatment journey.
Patient Resources

TAVALISSE Patient Information
Reviews important information for patients about TAVALISSE, including for whom TAVALISSE is intended, how to take TAVALISSE, and what to expect while on treatment.
PATIENT VIDEO LIBRARY

A Different Approach to Treatment
In this overview of treatment for chronic ITP, advanced nurse practitioner Charina Toste discusses how TAVALISSE works differently than other chronic ITP treatments. She explains that chronic ITP can be a serious condition when left untreated, but can be effectively managed with the right treatment.

What to Expect with TAVALISSE
Advanced nurse practitioner Charina Toste gives patients an overview of treatment with TAVALISSE and shares what to expect from their medication, which can help patients more clearly recognize progress toward their treatment goals.

Fitting TAVALISSE into Your Lifestyle
TAVALISSE is a medication with dosing flexibility, making it convenient for patients to fit it into their lifestyle. Advanced nurse practitioner Charina Toste provides tips to help patients get the benefits of treatment by taking their medication regularly as prescribed.

Setting Your Treatment Goals
Identifying personal goals—like getting back to activities they enjoy—and discussing them with their healthcare provider can help patients stay on track with treatment. Advanced nurse practitioner Charina Toste demonstrates how to set treatment goals and track progress.



