53-YEAR-OLD FEMALE WITH CHRONIC ITP, POST-STEROIDS

Sarah*
BACKGROUND
| AGE: |
53
|
| OCCUPATION: |
Baker
|
| DATE OF DIAGNOSIS: |
2004
|
- Metabolic syndrome
- Obesity
- Hypertension, controlled with nebivolol
- Hypothyroidism, controlled with levothyroxine
- Platelet Counts
- Treatment Summary
Sarah's Platelet Counts Over Time
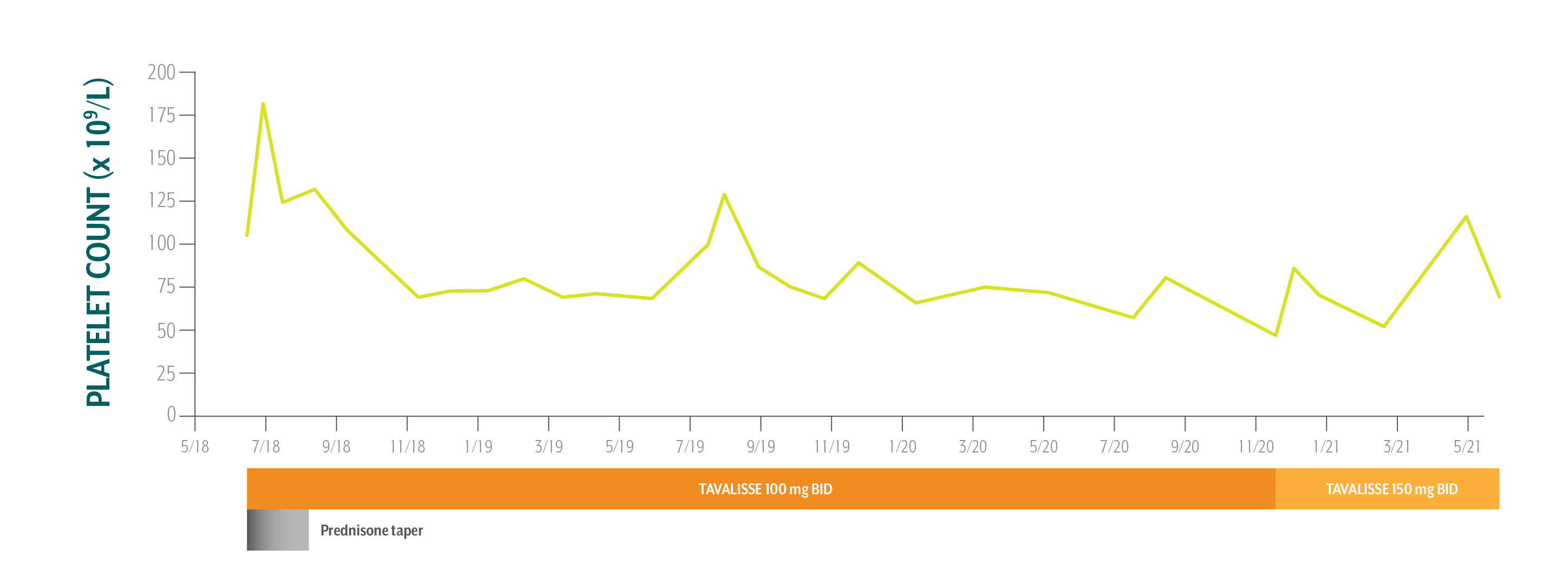
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
Sarah has been on TAVALISSE for 3 years and continues to achieve a clinical benefit. She is doing well on therapy and will continue with bimonthly visits.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BID=twice per day; BP=blood pressure; CBC=complete blood count; IVIG=intravenous immune globulin; QD=once per day.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Sarah's Treatment Summary
- Headings:
Prior Treatment history†
April 10, 2018: Sarah visits new hematologist/oncologist (hem-onc) upon referral from primary care physician due to upper respiratory infection and concern over decrease in platelet count
- Headings:
treatment with tavalisse
June 12, 2018: TAVALISSE initiation
- Platelet Count:
30 x 109/L
- Platelet Count:
105 x 109/L
- Clinical Observations:
- Negative for bruising/bleeding
- Clinical Observations:
Sarah has gained 14 lb since last visit (40 lb total since initiating prednisone in April)
- BP: 118/75
- Laboratory Findings:
N/A
- Laboratory Findings:
- ALT: 17 IU/L
- AST: 13 IU/L
- Bilirubin: 0.7 mg/dL
- Patient Discussion:
- Sarah is unwilling to continue steroids due to weight gain and wants to try a different treatment
- Hem-onc is also concerned about the potential adverse reactions of long-term steroid use and agrees to explore options
- Treatment options are presented and discussed with Sarah
- Sarah is averse to injections and infusions that would require her to travel a long distance to her hem-onc’s office
- Patient Discussion:
- Sarah and her hem-onc opted for treatment with TAVALISSE because it’s an oral treatment without food restrictions1
- Hem-onc counseled Sarah on the potential efficacy and adverse events of TAVALISSE and explained how the mechanism of TAVALISSE inhibits platelet destruction
- Treatment Plan:
- Treated with intermittent IVIG and steroids by transplant hematologist until 2013
- Platelet counts stabilized in 2013 and Sarah was transferred to primary care physician
- Primary care physician monitored Sarah until 2018, when she was referred to new hem-onc while experiencing an upper respiratory infection; primary care physician was concerned about a decrease in platelet count
- In July 2018, Sarah was treated with 3 doses of IV methylprednisolone over 3 days, followed by 2 months of prednisone 50 mg QD
- Despite sufficient platelet response, Sarah experienced substantial weight gain (40 lb in 2 months), which made her reluctant to continue treatment
Due to Sarah’s rapid weight gain, the treating physician sought a targeted treatment that could sustain healthy platelet counts while limiting the need for steroid use.
- Treatment Plan:
- Begin prednisone taper by reducing dose to 25 mg QD
- Initiate TAVALISSE at 100 mg BID and increase to 50 mg BID after week 4 if necessary
Sarah has been on TAVALISSE for 3 years and continues to achieve a clinical benefit. She is doing well on therapy and will continue with bimonthly visits.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BID=twice per day; BP=blood pressure; CBC=complete blood count; IVIG=intravenous immune globulin; QD=once per day.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
†Medical records prior to 2018 are unavailable.

PATIENT REFERRED TO NEW HEMATOLOGIST/ONCOLOGIST (HEM-ONC) FROM THE PRIMARY CARE PHYSICIAN WHO WAS MANAGING HER ITP