63-YEAR-OLD MALE WITH CHRONIC ITP AND CARDIOVASCULAR DISEASE

Daniel*
BACKGROUND
| AGE: |
63
|
| OCCUPATION: |
Lawyer
|
| DATE OF DIAGNOSIS: |
May 1, 2018
|
- Unprovoked deep-vein thrombosis (DVT) in 2014; now controlled
- Hypertension, controlled with bumetanide 1 mg QD, carvedilol 3.125 mg BID, losartan 25 mg QD
- Hyperlipidemia, controlled with atorvastatin 40 mg QD
- Diabetes mellitus type 2, controlled with empagliflozin 10 mg QD, glimepiride 4 mg QD, insulin glargine 20 U SC QD, linagliptin 5 mg QD, metformin 1 g QD
- Platelet Counts
- Treatment Summary
Daniel's Platelet Counts Over Time
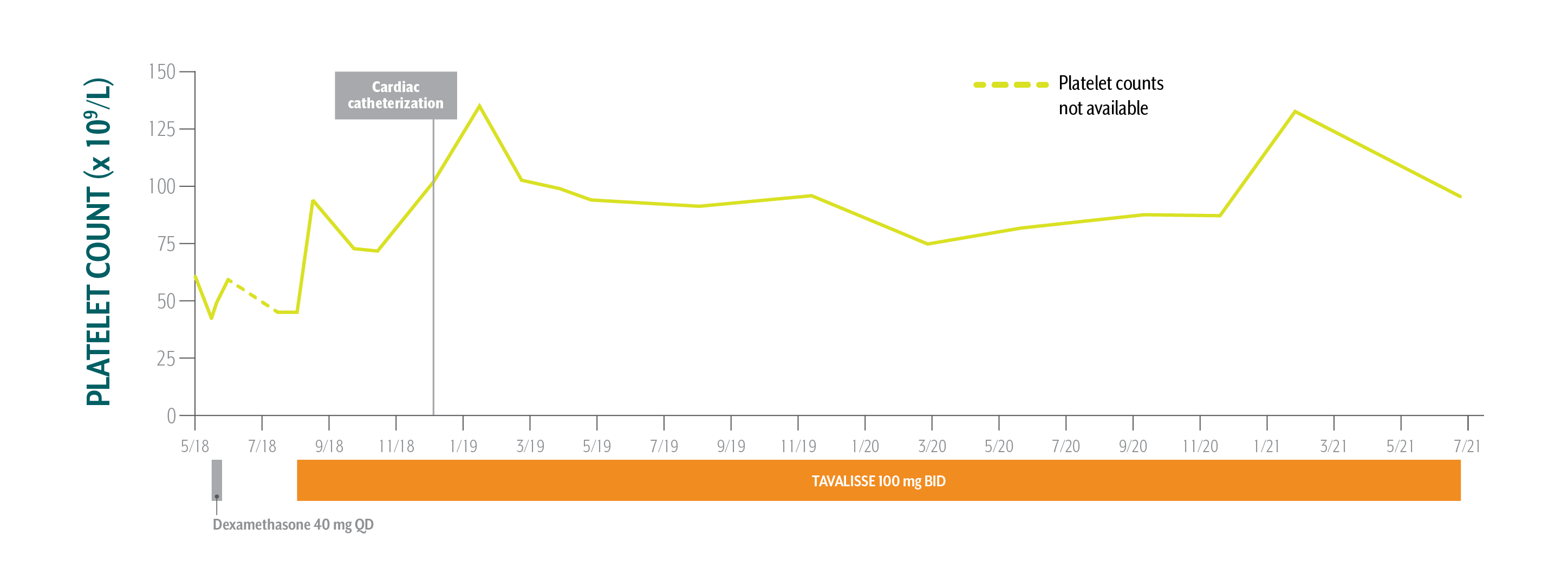
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
Patient has been taking TAVALISSE 100 mg BID for 3 years. Overall, he is very happy with his treatment. His platelet counts are stable, and he is doing well.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; CBC=complete blood count; Hb=hemoglobin; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Daniel's Treatment Summary
- Headings:
Prior Treatment history
May-July 2018: While under the care of a hematology/oncology (hem-onc) specialist for ITP, cardiac workup reveals a need for cardiac catheterization
- Headings:
treatment with tavalisse
August 1, 2018: TAVALISSE initiation
- Platelet Count:
43 x 109/L to 61 x 109/L (fluctuating)
- Platelet Count:
45 x 109/L
- Clinical Observations:
- Mild epistaxis, mild fatigue, easy bruising
- Clinical Observations:
- Patient is clinically stable with no bleeding events
- Laboratory Findings:
- Variable
- Laboratory Findings:
- WBC: 4.4 x 109/L
- Hb: 12.9 g/dL
- AST: 39 IU/L
- ALT: 27 IU/L
- Bilirubin: 1.1 mg/dL
- Negative for hepatitis B/C
- Patient Discussion:
- PCP diagnoses Daniel with thrombocytopenia and refers him to hem-onc for further treatment
- Despite symptoms subsiding with initial treatment, patient is informed that he will require further treatment for immune thrombocytopenia (ITP) stemming from his need for cardiovascular surgery
- Patient Discussion:
- Physician explained the need for rapid, robust platelet increase to ≥70 x 109/L for planned cardiac catheterization
- Physician explained the mechanism of TAVALISSE and the potential efficacy and adverse events
- Patient has an active lifestyle; patient and physician agree that an oral medication that can be taken with or without food would be a good fit
- Treatment Plan:
- Initiate dexamethasone 40 mg QD
- After it is determined that patient requires surgery, platelet target set at ≥70 x 109/L
In view of Daniel’s need of an increase in platelet counts for a planned cardiac catheterization, the absence of meaningful clinical response to first-line steroids, and his active lifestyle, the hem-onc sought an oral medication in a different class of therapy.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and increase to 150 mg BID after week 4 if necessary
Patient has been taking TAVALISSE 100 mg BID for 3 years. Overall, he is very happy with his treatment. His platelet counts are stable, and he is doing well.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; CBC=complete blood count; Hb=hemoglobin; WBC=white blood cell.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

PATIENT WITH MILD EPISTAXIS, EASY BRUISING, AND MILD FATIGUE REFERRED TO HEMATOLOGY/ONCOLOGY SPECIALIST (HEM-ONC) BY PRIMARY CARE PHYSICIAN (PCP)