37-YEAR-OLD MALE WITH ITP AND A HISTORY OF GERD

Samuel*
BACKGROUND
| AGE: |
37
|
| OCCUPATION: |
Taxi driver
|
| DATE OF DIAGNOSIS: |
December 2019
|
- Gastroesophageal reflux disease (GERD) diagnosed in 2016; partially controlled with diet and antacids as needed
- Platelet Counts
- Treatment Summary
Samuel's Platelet Counts Over Time
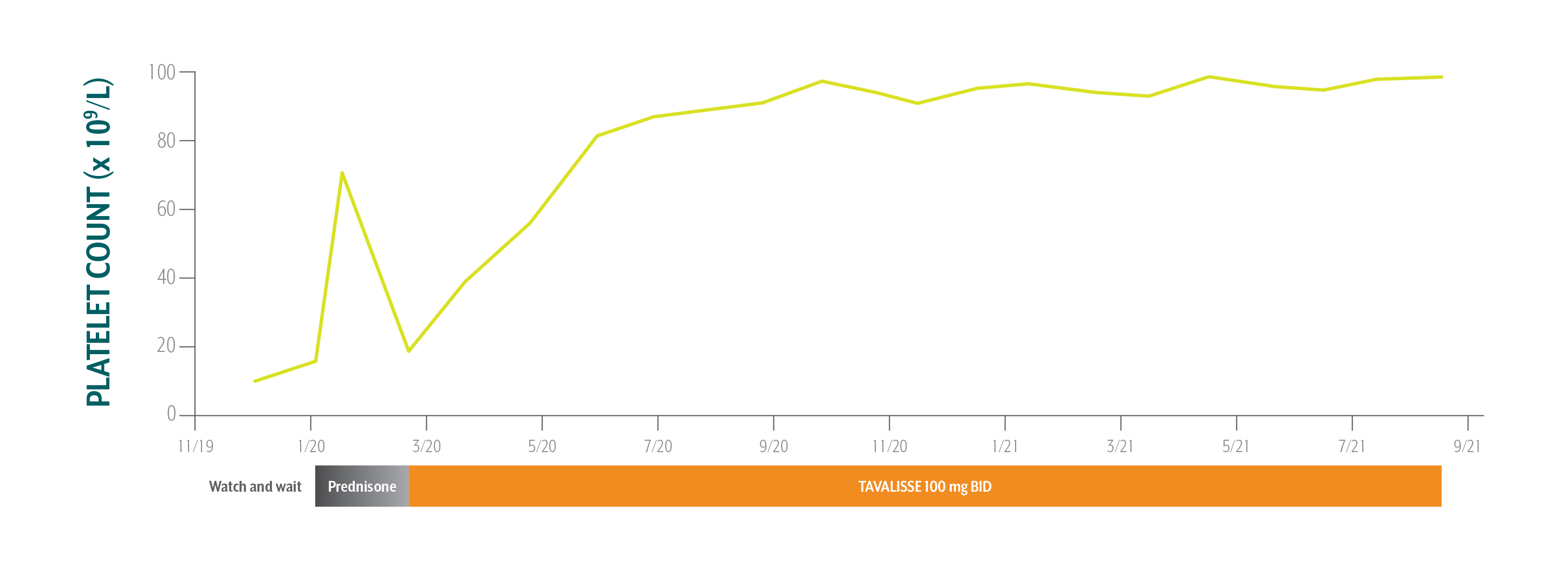
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
The patient is pleased with his stable and consistent increase in platelet counts while on TAVALISSE. He is happy that he does not have to follow up more than once a month and that he can take it with or without food.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; Hb=hemoglobin; TPO-RA=thrombopoietin receptor agonist; WBC=white blood cell; WNL=within normal limits.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Samuel's Treatment Summary
- Headings:
Prior Treatment history
December 2, 2019: PCP referred patient to hem-onc after ITP diagnosis
- Headings:
treatment with tavalisse
February 21, 2020: TAVALISSE initiation
- Platelet Count:
10 x 109/L
- Platelet Count:
19 x 109/L
- Clinical Observations:
- Fatigue, anxiety, bone pain, bruising
- Clinical Observations:
- Fatigue, bruising on the lower extremities, bone pain
- Laboratory Findings:
- Hb: 14.9 g/dL
- WBC: 3.0 x 109/L
- AST: 23 IU/L
- ALT: 23 IU/L
- Bilirubin: 1.1 mg/dL
- BP: 118/74 mm Hg
- Laboratory Findings:
- Hb: 14.3 g/dL
- WBC: 4.1 x 109/L
- AST: 29 IU/L
- ALT: 32 IU/L
- Bilirubin: 0.8 mg/dL
- BP: 117/73 mm Hg
- Patient Discussion:
- PCP diagnosed Samuel with ITP and referred him to hem-onc for further treatment
- Patient stated he had intermittent epistaxis in the past; hem-onc explained that this could be caused by ITP and that treatment to prevent bleeding is necessary
- Patient leads an active lifestyle and expressed aversion to taking additional medication
- Patient Discussion:
- Patient is opposed to taking medications and requested vitamins; physician explained that his choices were injections or oral medications
- Patient wanted to avoid injections and stated that it is difficult for him to come to the office frequently because of his job and active lifestyle
- Physician discussed the mechanisms of TAVALISSE and TPO-RAs; patient liked TAVALISSE because “it reduces the destruction of platelets” and has no food restrictions1
- Physician explained the potential efficacy and adverse events of TAVALISSE; patient stated he was willing to take medication daily as long as it helps
- Treatment Plan:
- Treat with prednisone to increase platelet count
Based on Samuel’s history of GERD, active lifestyle, and inability to visit the office weekly, the hem-onc sought an oral medication with a targeted mechanism that could sustain stable platelet counts while limiting the need for steroid use.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and increase to 150 mg BID after week 4 if necessary
The patient is pleased with his stable and consistent increase in platelet counts while on TAVALISSE. He is happy that he does not have to follow up more than once a month and that he can take it with or without food.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; Hb=hemoglobin; TPO-RA=thrombopoietin receptor agonist; WBC=white blood cell; WNL=within normal limits.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

PATIENT WITH FATIGUE, ANXIETY, BONE PAIN, AND BRUISING WAS DIAGNOSED WITH ITP BY PRIMARY CARE PHYSICIAN (PCP) AND REFERRED TO HEMATOLOGY/ONCOLOGY SPECIALIST (HEM-ONC)