TAVALISSE offers the convenience of oral dosing without food restrictions
TAVALISSE IS AN ORAL MEDICATION TAKEN TWICE DAILY WITH OR WITHOUT FOOD1
A 12-week evaluation period is recommended1,*
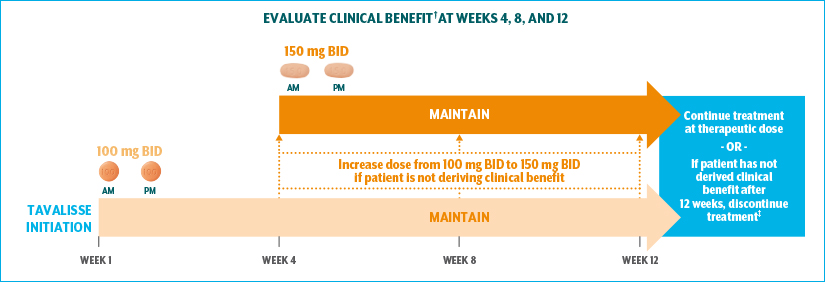
*12-week evaluation period recommended per product labeling.
†Clinical benefit: platelet count increase to a level sufficient to avoid clinically important bleeding.
‡Discontinue TAVALISSE after 12 weeks of treatment if the platelet count does not increase to a level sufficient to avoid clinically important bleeding.
88% of patients in the phase 3 clinical studies were maintained at the 150-mg BID dose

- TAVALISSE is available as 100-mg and 150-mg tablets
- If a dose is missed, the next dose should be taken at its regularly scheduled time
- In clinical trials, stable concurrent ITP therapy was allowed and rescue therapy was permitted if needed
- Use the lowest dose of TAVALISSE to achieve and maintain a platelet count of at least 50 x 109/L as necessary to reduce the risk of bleeding
Patient response may vary—adjust dose to achieve target platelet count
Recommended patient monitoring
As part of chronic ITP care and treatment with TAVALISSE, patient monitoring is recommended1

TAVALISSE dose modification guidance1
Modification of the TAVALISSE dose can help in the management of adverse reactions
- In addition to supportive care measures, management of some adverse reactions may require dose interruption, reduction, or discontinuation
- See section 2.3 of the full Prescribing Information for specific guidance on dose modifications and recommendations for treatment discontinuations regarding hypertension, hepatotoxicity, diarrhea, and neutropenia
- Starting with the current dose, gradually reduce the dose following the steps provided below until the adverse reaction resolves

- Concomitant use with a strong CYP3A4 inhibitor increases exposure to the major active metabolite of TAVALISSE. Monitor for toxicities of TAVALISSE that may require TAVALISSE dose modifications when given concurrently with a strong CYP3A4 inhibitor
§If further dose reduction below 100 mg/day is required, discontinue TAVALISSE.
||Once-daily TAVALISSE should be taken in the morning.
Reference: 1. TAVALISSE® [package insert]. South San Francisco, CA: Rigel Pharmaceuticals, Inc.; November 2020.
