TAVALISSE DELIVERED ROBUST, DURABLE, AND STABLE INCREASES IN PLATELET COUNTS
Response rates achieved in the 24-week evaluation period (double-blind, placebo-controlled trials)
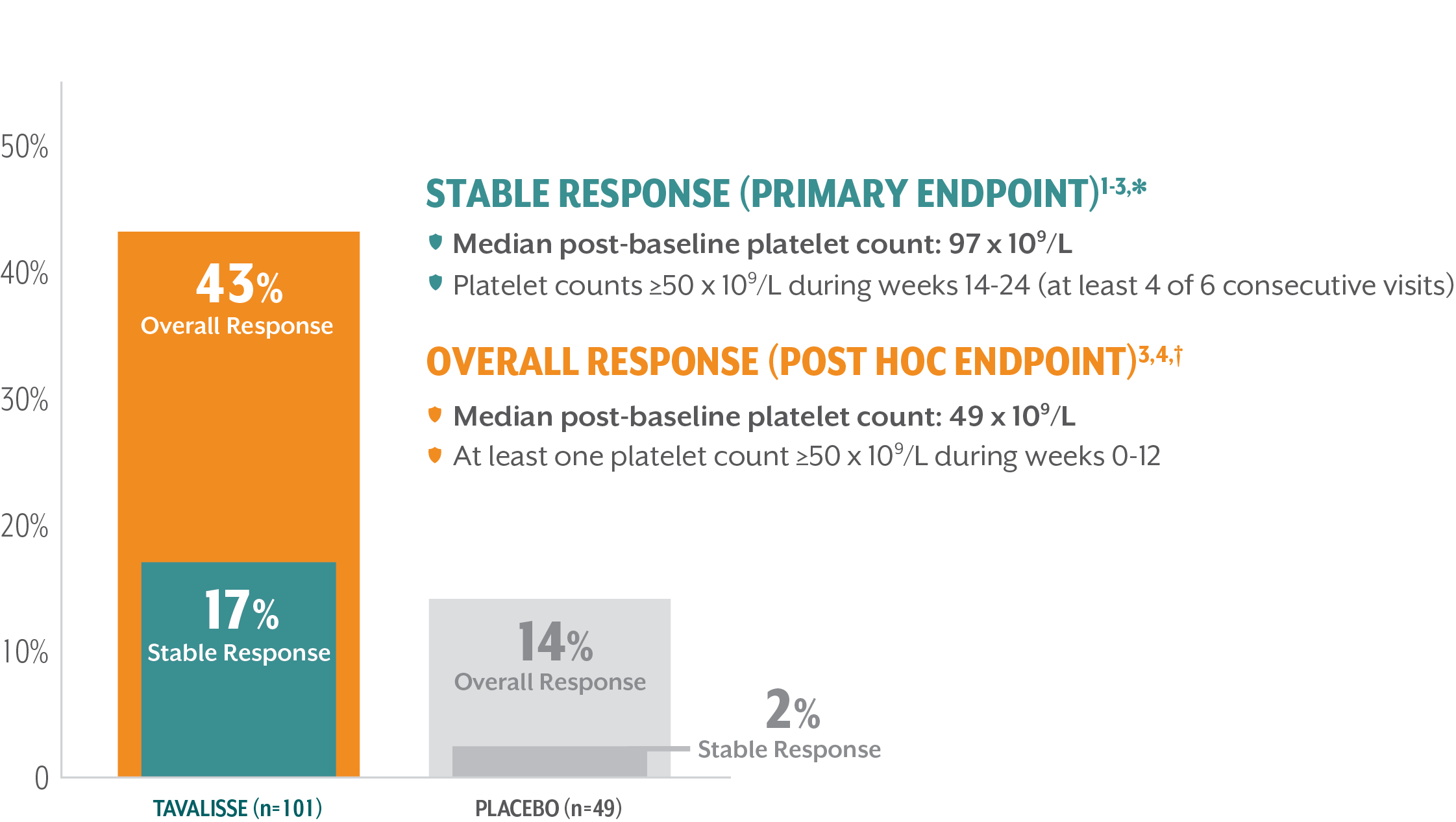
Median time to first response: 15 days4,‡
*Stable platelet response: achievement of a platelet count ≥50 x 109/L on ≥4 of the 6 visits during weeks 14-24 without need for rescue treatment.
†Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.
‡Time to first response was a post hoc analysis.
SUSTAINED CLINICAL BENEFIT WAS SEEN WITH LONG-TERM USE OF TAVALISSE
This 5-year interim analysis assessed response by the proportion of patients achieving platelet count thresholds over time5
Overall Response — those patients who achieved platelet counts ≥50 x 109/L during the first 12 weeks of treatment5,§
Clinical Benefit — those patients with platelet counts exceeding ≥30 x 109/L who were not classified as overall responders, because 50 x 109/L was not reached by week 121
Limited/No Benefit — those patients with platelet counts <30 x 109/L5
§Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.
TAVALISSE DEMONSTRATED A DURABLE, STABLE RESPONSE IN A 5-YEAR ANALYSIS
For most patients, once a response was achieved, it was maintained for the majority of time on treatment5,6,||,¶
In patients who responded, the median platelet count increased to between 50 x 109/L and 150 x 109/L for the duration of treatment5
||Patients were evaluated every 2 weeks in the placebo-controlled trials (FIT-1 + FIT-2) and no less often than monthly for 18 months, and then bimonthly for up to 5 years in the open-label extension study (FIT-3).2
¶A total of 102 patients exited the placebo-controlled trials early (at or after week 12) and entered the open-label extension study (FIT-3).1,4
#Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.4
**1 month = 4 weeks. Data cutoff date: December 2019.5
ROBUST IMPROVEMENTS IN PLATELET COUNTS WERE OBSERVED AFTER PRIOR STEROID AND/OR TPO-RA USE
A durable improvement in platelet counts was seen across all lines of therapy6
Earlier-line use of TAVALISSE resulted in higher rates of response6
Time to response (platelet count ≥50 x 109/L) in responders to TAVALISSE6:
- 56% of 2nd-line patients responded within 4 weeks
- 76% of 2nd-line patients responded within 12 weeks
- 81% of patients across all lines of therapy responded within 12 weeks
TPO-RA=thrombopoietin receptor agonist.
††Data cutoff date: December 2019.6
‡‡Platelet response was assessed by the proportion of patients achieving platelet counts of ≥50 x 109/L and of ≥30 x 109/L at any visit (without rescue therapy within 4 weeks).6
§§Percentage of patients achieving platelet counts of ≥30 x 109/L calculated using data on file.3
TAVALISSE demonstrated efficacy across patient subgroups compared to placebo
Treatment benefit favored TAVALISSE in the phase 3 clinical trials (FIT-1 + FIT-2)3,5
||||Post hoc subgroup.
TAVALISSE PATIENTS HAD FEWER BLEEDING EPISODES AND REQUIRED LESS RESCUE MEDICATION COMPARED TO PLACEBO
TAVALISSE patients had a lower incidence of bleeding episodes than placebo patients1,3
- In the placebo-controlled trials, the incidence of bleeding in patients who received TAVALISSE (regardless of responder status) was 29%, compared with 37% in patients who received placebo
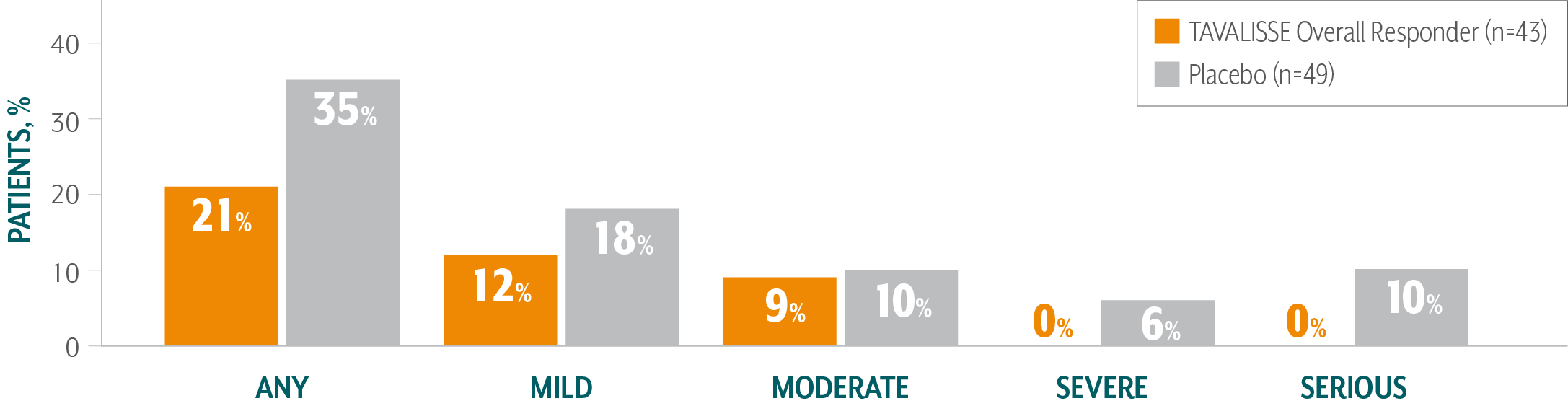
Of patients treated with TAVALISSE in the phase 3 clinical studies (FIT-1 + FIT-2 + FIT-3), 59% had no bleeding episodes3,¶¶
TAVALISSE patients required less rescue medication than placebo patients4
- In the placebo-controlled trials, 30% of patients who received TAVALISSE (regardless of responder status) required rescue medication, compared with 45% of patients who received placebo
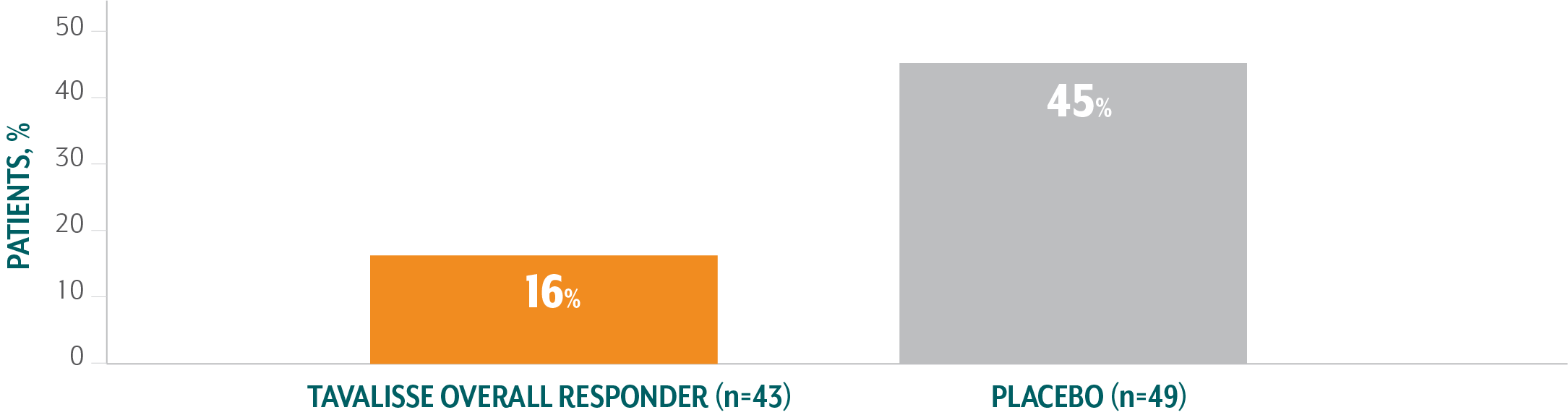
Of patients treated with TAVALISSE in the phase 3 clinical studies (FIT-1 + FIT-2 + FIT-3), 59% did not require rescue medication at any visit3,¶¶
¶¶Compiled from interim analysis. Data cutoff: March 8, 2018.
References: 1. TAVALISSE®. Package insert. Rigel Pharmaceuticals, Inc. 2. Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546-553. doi:10.1002/ajh.25444 3. Data on file, Rigel Pharmaceuticals, Inc. April 2018. 4. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. 5. Cooper N, Altomare I, Thomas MR, et al. Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12:1-12. doi:10.1177/20406207211010875 6. Boccia R, Cooper N, Ghanima W, et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol. 2020;190(6):933-938. doi:10.1111/bjh.16959
