68-YEAR-OLD MALE WITH REFRACTORY CHRONIC ITP

Steve*
BACKGROUND
| AGE: |
68
|
| OCCUPATION: |
Retired teacher
|
| DATE OF DIAGNOSIS: |
April 28, 2018
|
- AFib, controlled with aspirin
- Hypertension, controlled with metoprolol and lisinopril
- CAD, post-angioplasty
- Platelet Counts
- Treatment Summary
Steve's Platelet Counts Over Time
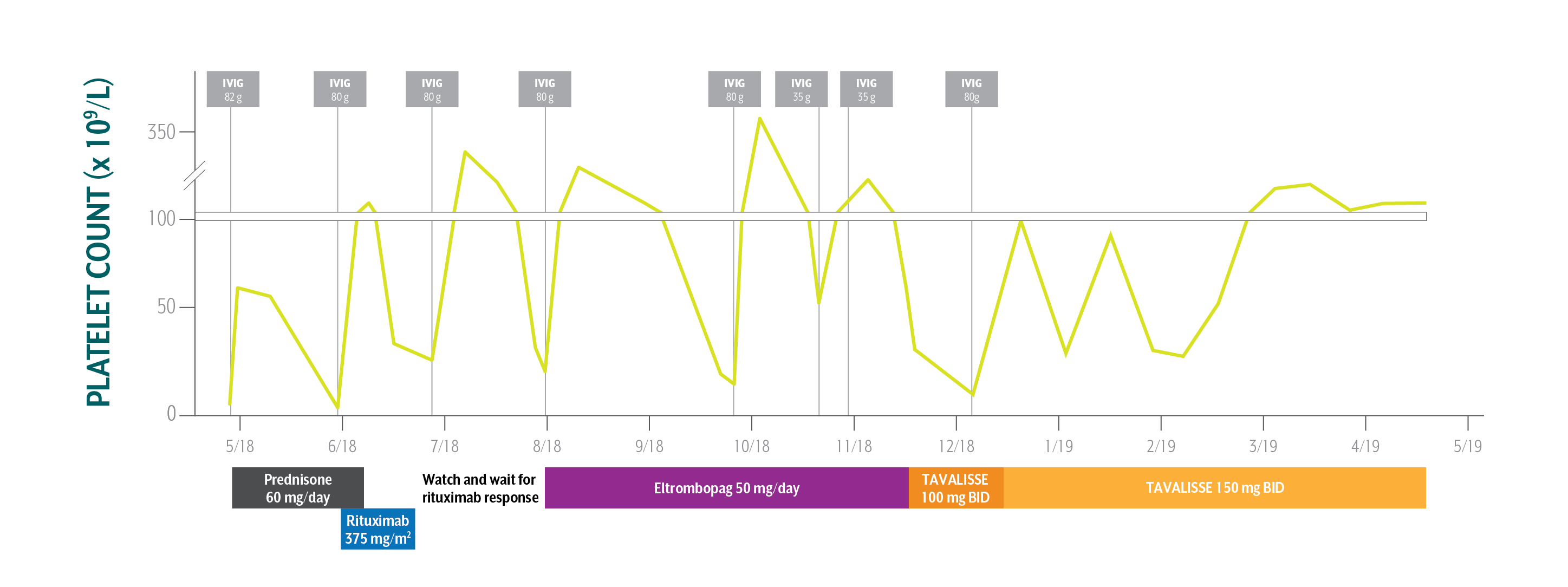
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
Although Steve’s initial response to TAVALISSE was gradual, his platelet count increased and response was maintained.
AFib=atrial fibrillation; CAD=coronary artery disease; IVIG=intravenous immunoglobulin.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Steve's Treatment Summary
- Headings:
Prior Treatment history
April 28, 2018: Upon admission to the emergency room, ER team assesses bleeding
- Headings:
treatment with tavalisse
November 16, 2018: TAVALISSE initiation
- Platelet Count:
<5 x 109/L
- Platelet Count:
42 x 109/L (following IVIG)
- Clinical Observations:
- Petechiae on extremities; oral bleeding; right-sided epistaxis; ER doctor suspects ITP
- Clinical Observations:
- Negative for bleeding/bruising
- Laboratory Findings:
- Red blood count (RBC): 3.56 million/µL
- Hemoglobin (Hb): 12.2 g/dL
- Hematocrit: 35.4%
- Mean corpuscular volume (MCV): 99.4 fL/cell
- Mean cell hemoglobin (MCH): 34.3 pg/cell
- Mean cell hemoglobin concentration (MCHC): 34.5 g/dL
- Red cell distribution width (RDW): 13.1%
- White blood count: 5.93 x 109/L
- Neutrophils: 3.45 x 109/L
- Lymphocytes: 1.78 x 109/L
- Monocytes: 0.49 x 109/L
- Eosinophils: 0.16 x 109/L
- Laboratory Findings:
- All labs normal
- ER Treatment:
- Transfused with single-donor platelet pack upon admission (no improvement in platelet count)
- Prescribed prednisone 60 mg/day and instructed to discontinue aspirin
- ER Treatment:
N/A
- Patient Discussion:
- ER doctor referred Steve to local hematologist/oncologist (hem-onc) to manage treatment; hem-onc confirmed ITP diagnosis
- Patient Discussion:
- Physician explained the mechanism of TAVALISSE and the potential efficacy and adverse events
- Physician counseled Steve on how to manage diarrhea, should it occur
- Steve is pleased to remain on an oral medication that doesn’t require weekly visits
- Treatment Plan:
- Physician administered IVIG 82 g
- Plan is to initiate prednisone 60 mg/day
- If platelet count doesn’t stabilize, physician to treat with 6 weekly infusions of rituximab, observe, and treat with eltrombopag
- Splenectomy is presented as a treatment option—Steve is unwilling to undergo surgery
After multiple therapies resulting in consistent decreases in Steve’s platelet counts, the treating physician sought a different class of therapy.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and increase to 150 mg BID after week 4 if necessary
Although Steve’s initial response to TAVALISSE was gradual, his platelet count increased and response was maintained.
AFib=atrial fibrillation; CAD=coronary artery disease; IVIG=intravenous immunoglobulin.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

PATIENT ADMITTED TO EMERGENCY ROOM WITH BLEEDING