35-YEAR-OLD FEMALE WITH ITP

Nicole*
BACKGROUND
| AGE: |
35
|
| OCCUPATION: |
Unemployed
|
| DATE OF DIAGNOSIS: |
June 20, 2018
|
- Depression, controlled with fluoxetine 20 mg QD
- Anxiety, controlled with lorazepam 2 mg TID
- Bipolar disorder, controlled with aripiprazole 10 mg QD
- Diagnosed with hepatitis C, treated with ledipasvir/sofosbuvir (PCR negative)
- History of thrombocytopenia during pregnancy (2015)
- History of polysubstance abuse (clean for 3 years)
- Platelet Counts
- Treatment Summary
Nicole's Platelet Counts Over Time
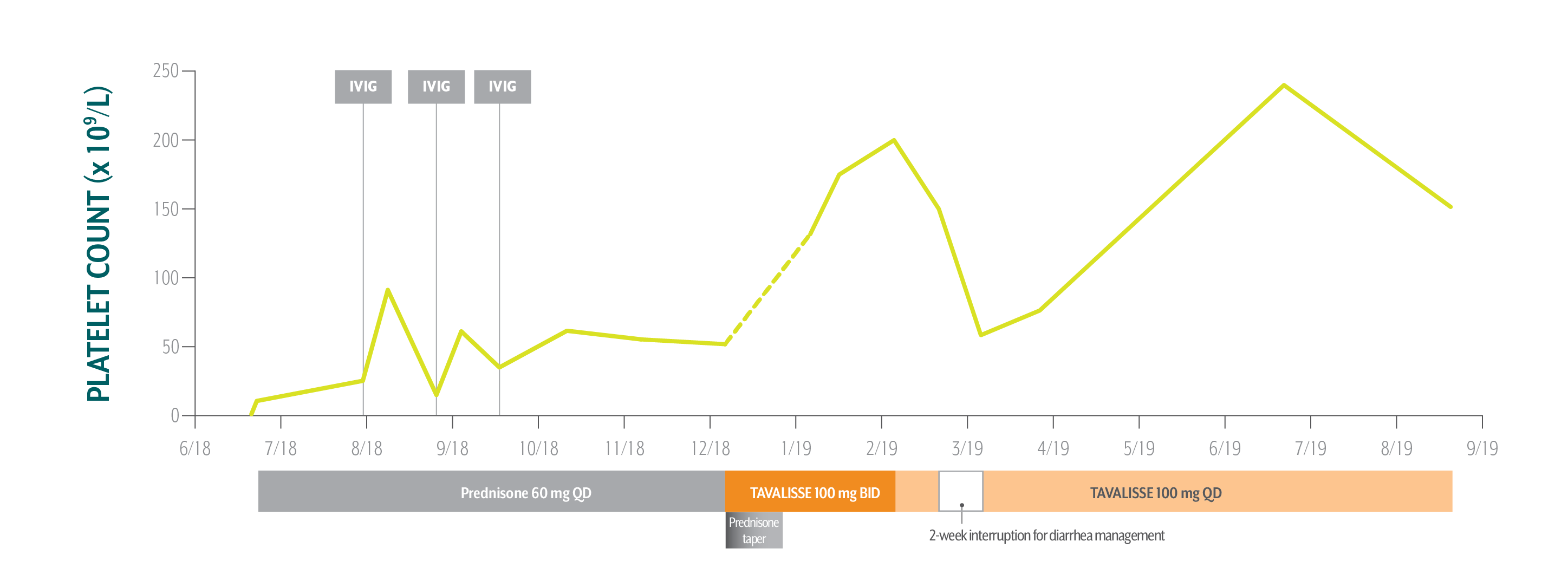
Swipe/click right to explore the details for each time point
- Date
- Date Platelet Count
- Clinical Observations
- Laboratory Findings
- Physician Notes
- Treatment Plan
Nicole responded well to TAVALISSE—her platelet count stabilized above 150 x 109/L on the 100 mg QD dose and she hasn’t experienced any bleeding.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; IVIG=IV immunoglobulin; LFT=liver function test.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.
Nicole's Treatment Summary
- Headings:
Prior Treatment history
June 20, 2018: Patient referred to hematology/oncology specialist by primary care physician suspecting ITP due to severe bruising on extremities
- Headings:
treatment with tavalisse
December 5, 2018: TAVALISSE initiation
- Platelet Count:
4 x 109/L
- Platelet Count:
52 x 109/L
- Clinical Observations:
- Severe bruising on arms and legs
- Clinical Observations:
- Negative for bleeding/bruising
- BP: 123/73
- Laboratory Findings:
- Normal
- Laboratory Findings:
- Bilirubin: 0.8 mg/dL
- AST: 21 IU/L
- ALT: 18 IU/L
- Patient Discussion:
- Nicole shared that she has a history of intravenous (IV) drug use and that she hasn’t used drugs in 3 years; she would like to avoid injections or infusions for this reason
- Nicole recalls experiencing transient thrombocytopenia during pregnancy (2015)
- Patient Discussion:
- While discussing treatment options, Nicole expressed preference for an oral medication due to history of IV drug use and a desire for less frequent office visits
- TAVALISSE was selected because it is an oral medication in a different class of therapy
- Hem-onc counseled Nicole on the potential efficacy and adverse events of TAVALISSE and explained how the mechanism of TAVALISSE inhibits platelet destruction
- Hem-onc counseled Nicole on how to manage diarrhea, should it occur
- Treatment Plan:
- Initiate prednisone 60 mg QD; Nicole’s low platelet count and symptoms require treatment to rapidly increase platelet counts
After Nicole experienced transient responses to IVIG while on prednisone, her hem-onc sought an oral medication in a different class of therapy.
- Treatment Plan:
- Initiate TAVALISSE 100 mg BID and titrate dose as needed; taper prednisone over 3 weeks, then discontinue
Nicole responded well to TAVALISSE—her platelet count stabilized above 150 x 109/L on the 100 mg QD dose and she hasn’t experienced any bleeding.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; BP=blood pressure; IVIG=intravenous immunogobulin; LFT=liver function test.
*This case study contains data from an actual TAVALISSE patient. Patient name and image have been changed to protect privacy. This case study is intended for general medical education purposes only and is not a substitute for independent clinical medical judgment. The intent of this case study is to present the experience of a single patient, which may not represent the outcomes in the overall patient population. Response to treatment may vary from patient to patient.

PATIENT REFERRED TO HEMATOLOGY/ONCOLOGY SPECIALIST (HEM-ONC) BY PRIMARY CARE PHYSICIAN SUSPECTING ITP DUE TO SEVERE BRUISING ON EXTREMITIES