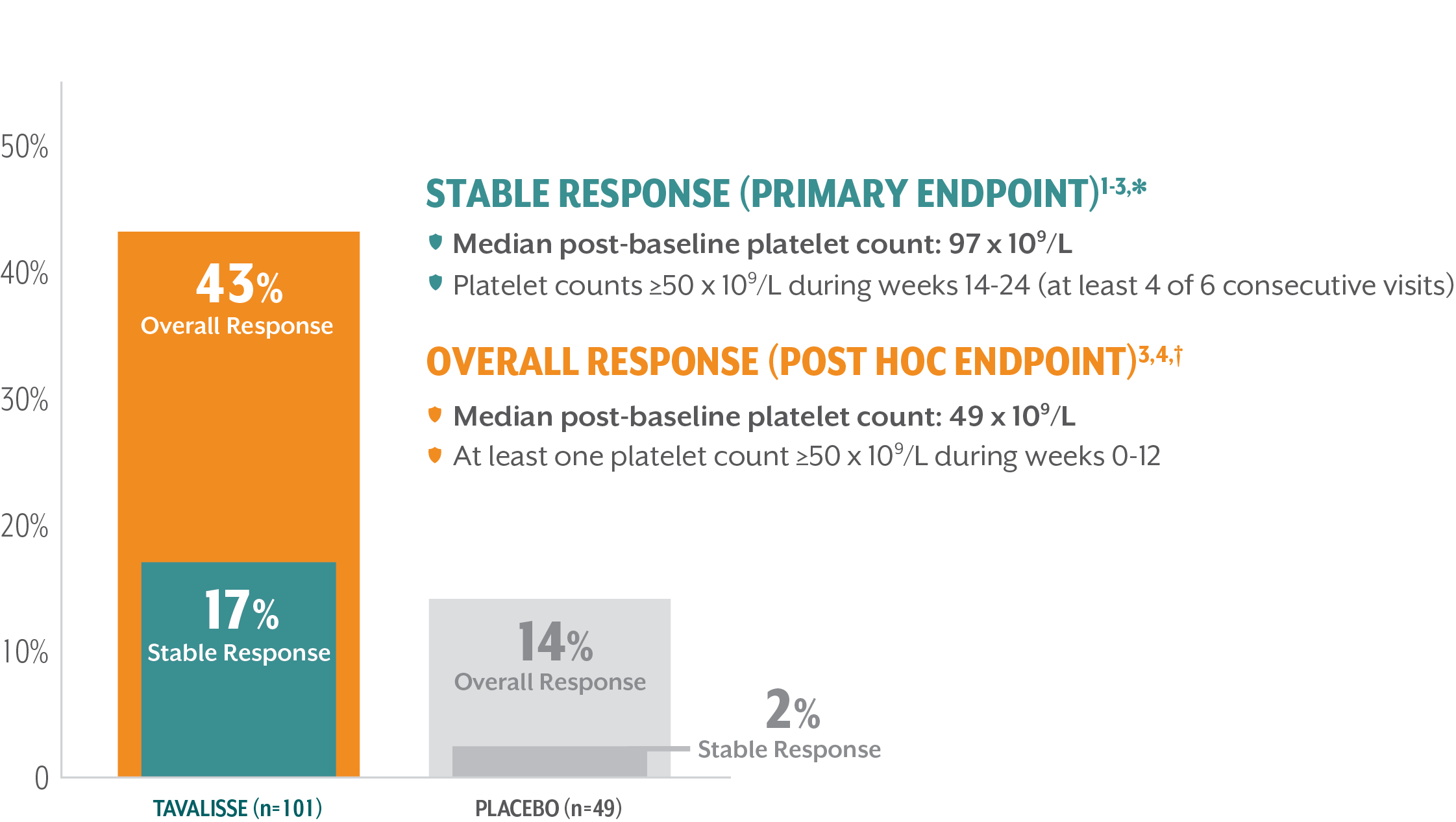
TAVALISSE delivered rapid, robust, and durable improvements in platelet counts
Response rates achieved in the 24-week evaluation period (double-blind, placebo-controlled trials)
Median time to first response: 15 days4,‡
*Stable platelet response: achievement of a platelet count ≥50 x 109/L on ≥4 of the 6 visits during weeks 14-24 without need for rescue treatment.
†Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.
‡Time to first response was a post hoc analysis.
TAVALISSE DELIVERED ROBUST IMPROVEMENTS IN PLATELET COUNTS5
This 5-year interim analysis assessed response by the proportion of patients achieving platelet count thresholds over time
Overall Response — those patients who achieved platelet counts ≥50 x 109/L during the first 12 weeks of treatment§
Clinical Benefit — those patients with platelet counts exceeding ≥30 x 109/L who were not classified as Overall Responders, because 50 x 109/L was not reached by week 12∥
Limited/No Benefit — those patients with platelet counts <30 x 109/L
§Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.
∥Patients achieving Clinical Benefit achieved platelet counts between 30 x 109/L and 49 x 109/L within the first 12 weeks of treatment.
FIVE-YEAR EFFICACY ANALYSIS: TAVALISSE DEMONSTRATED DURABLE BENEFIT5
Durable efficacy was observed in responders to TAVALISSE in the FIT studies (combined results from FIT-1 + FIT-2 + FIT-3)¶,#
While patients achieving a Clinical Benefit had a more gradual response to treatment, TAVALISSE demonstrated a durable response, with median post-baseline platelet counts that on average stabilized between 30 x 109/L and 50 x 109/L
¶Patients were evaluated every 2 weeks in the placebo-controlled trials (FIT-1 + FIT-2) and no less often than monthly for 18 months, and then bimonthly for up to 5 years in the open-label extension study (FIT-3).
#A total of 102 patients exited the placebo-controlled trials early (at or after week 12) and entered the open-label extension study (FIT-3).
**Overall response: achievement of a platelet count ≥50 x 109/L at least once during the first 3 months/12 weeks without need for rescue treatment.
††1 month = 4 weeks. Data cutoff date: December 2019.
RESPONSE TO TAVALISSE WAS SEEN IN PATIENTS ACROSS ALL LINES OF THERAPY
A post hoc analysis of the phase 3 clinical studies was conducted to evaluate patient response by line of therapy
Higher response rates were seen in patients exposed only to steroids (with or without immunoglobulins) prior to receiving TAVALISSE
Time to response (platelet count ≥50 x 109/L) in responders to TAVALISSE6:
- 56% of 2nd-line patients responded within 4 weeks
- 76% of 2nd-line patients responded within 12 weeks
- 81% of patients across all lines of therapy responded within 12 weeks
‡‡Data cutoff date: December 2019.
§§Platelet response was assessed by the proportion of patients achieving platelet counts of ≥50 x 109/L and of ≥30 x 109/L at any visit (without rescue therapy within 4 weeks).
¶¶Percentage of patients achieving platelet counts of ≥30 x 109/L calculated using data on file.3
This analysis measured the percentage of treatment days that patients maintained their response
“Although rates of response varied, response, once achieved, was maintained (durable) irrespective of number of prior lines of therapy.” —Boccia et al. Br J Haematol. 2020.6
The majority of 2nd-line patients with platelet counts ≥50 x 109/L maintained their response for >36 months6
- Rates of adverse events in these subgroups were consistent with those in patients treated with TAVALISSE in the placebo-controlled trials4,6
||||Durability of response: median percentage of treatment days that patients maintained a response of ≥50 x 109/L or ≥30 x 109/L, with loss of response at the first of two platelet counts ≤30 x 109/L or ≤20 x 109/L, respectively, at least 4 weeks apart, or use of rescue therapy.
TAVALISSE demonstrated efficacy across patient subgroups compared to placebo5
Treatment benefit favored TAVALISSE in the phase 3 clinical trials (FIT-1 + FIT-2)3
¶¶Post hoc subgroup.
TAVALISSE PATIENTS HAD FEWER BLEEDING EPISODES AND REQUIRED LESS RESCUE MEDICATION COMPARED TO PLACEBO
TAVALISSE patients had a lower incidence of bleeding episodes than placebo patients1,3
- In the placebo-controlled trials, the incidence of bleeding in patients who received TAVALISSE (regardless of responder status) was 29%, compared with 37% in patients who received placebo
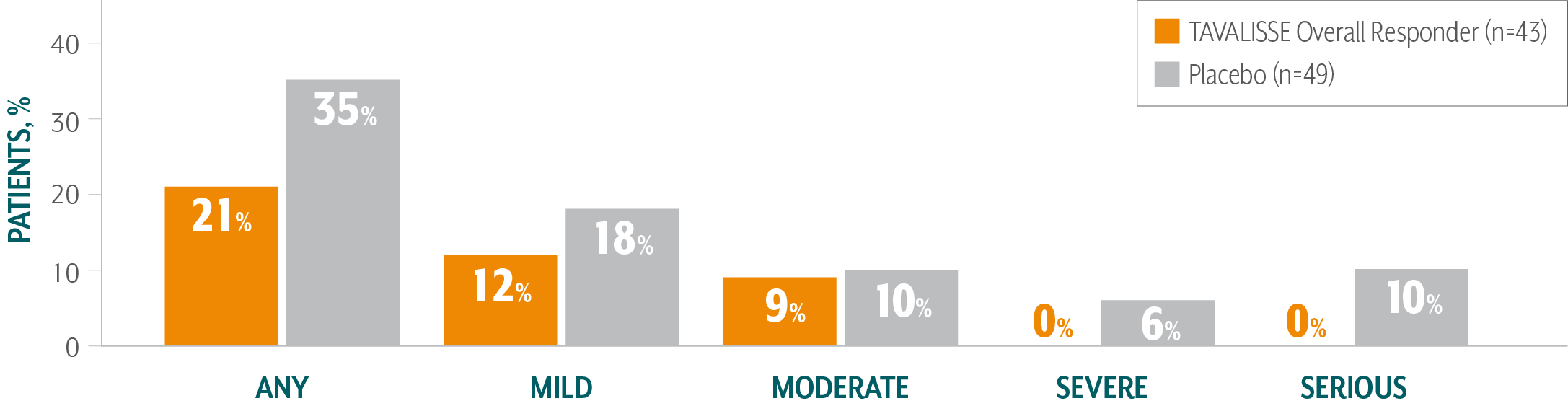
Of patients treated with TAVALISSE in the phase 3 clinical studies (FIT-1 + FIT-2 + FIT-3), 59% had no bleeding episodes.3,##
TAVALISSE patients required less rescue medication than placebo patients4
- In the placebo-controlled trials, 30% of patients who received TAVALISSE (regardless of responder status) required rescue medication, compared with 45% of patients who received placebo
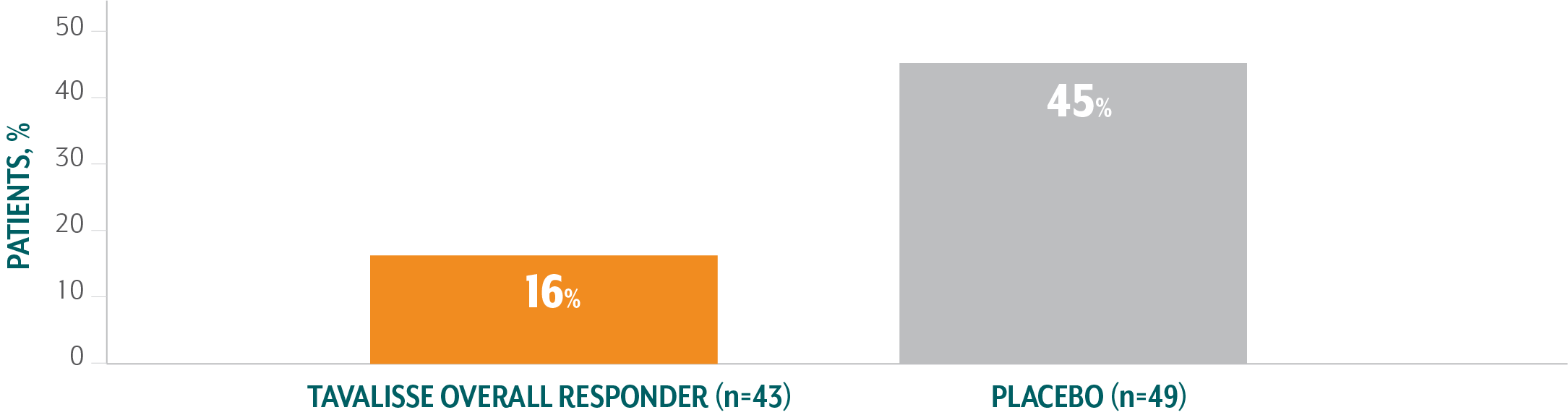
Of patients treated with TAVALISSE in the phase 3 clinical studies (FIT-1 + FIT-2 + FIT-3), 59% did not require rescue medication at any visit.3,§§
##Compiled from interim analysis. Data cutoff: March 8, 2018.
References: 1. TAVALISSE® [package insert]. South San Francisco, CA: Rigel Pharmaceuticals, Inc.; November 2020. 2. Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546-553. 3. Data on file, Rigel Pharmaceuticals, Inc. April 2018. 4. Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. 5. Cooper N, Altomare I, Thomas MR, et al. Assessment of thrombotic risk during long-term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12:e20406207211010875. doi:10.1177/20406207211010875 6. Boccia R, Cooper N, Ghanima W, et al. Fostamatinib is an effective second-line therapy in patients with immune thrombocytopenia. Br J Haematol. 2020;190(6):933-938.
